Stem Cell Therapy

Mesenchymal stem cell (MSC) therapy utilises human stem cells to help repair, regenerate and replenish damaged body tissues or organs in the body.
It is a less-invasive procedure and can also be administered directly to target sites, depending on the patient’s disease or condition. This makes it different from conventional treatment, as only isolated stem cells are infused into the patient and not whole tissues or organs.
The potential healing properties of MSC therapy include, but are not limited to, regenerating nerves, regrowing cartilage, reducing inflammation and repairing damaged tissues.
How Does
Stem Cell Therapy Work?
MSCs have an intrinsic property which attracts them to inflammation sites in the body. The stem cells then help to regenerate damaged or diseased tissues, reduce inflammation and modulate the immune system.
These stem cells influence tissue repair through cell signalling or direct cell-to-cell contact, in order to change the behaviour of existing cells and induce positive changes in the body.
Medical Conditions
That Could Benefits from Stem Cell Therapy
Studies have shown that with MSC therapy, patients have seen regenerative improvements associated with symptoms of diseases and conditions, such as those listed below.
- Amyotrophic lateral sclerosis (ALS)
- Alzheimer’s disease
- Arthritis
- Avascular necrosis
- Chronic obstructive pulmonary disease
- Crohn’s disease
- Degenerative disc disease
- Diabetes
- Erectiledysfunction Hair loss
- Hearing loss
- Heart disease
- Kidney failure
- Knee injury
- Liver disease
- Lung disease
- Macular degeneration
- Multiple sclerosis
- Muscular dystrophy
- Myasthenia gravis
- Non-union fracture
- Optic nerve degeneration
- Osteoarthritis
- Parkinson’s disease
- Psoriasis
- Rotator cuff
- Scleroderma
- Skin burn
- Spinal cord injuries
- Torn ligaments
- Tennis elbow
- Tendinitis
- Stroke
- Vasculitis

Advantages
Compared to other types of stem cells, MSCs have the following advantages:
1. Diverse Differentiation Potential
MSCs can be differentiated into a variety of cell types in the laboratory, including but not limited to nerve, tendon, ligament, bone, skin, fat, cartilage, muscle and marrow stroma cells.
2. Immune Privileged
MSCs have shown to not elicit a negative response from a person’s immune system. This allows the cells to be transplanted into the body without rejection, making them a universal source of stem cells.
3. Ethical & Legal
MSCs avoid ethical issues surrounding embryonic stem cells, as they can be derived from readily available sources that include donated umbilical cord tissues.
4. Flexible Propagation
MSCs can be grown and propagated in culture for extended periods of time, without losing their differentiation potential
5. Large Scale Expansion
MSCs can be easily expanded, in accordance with Current Good Manufacturing Process (cGMP) requirements, to a desired amount of cells for clinical applications.
Source of Our Stem Cell
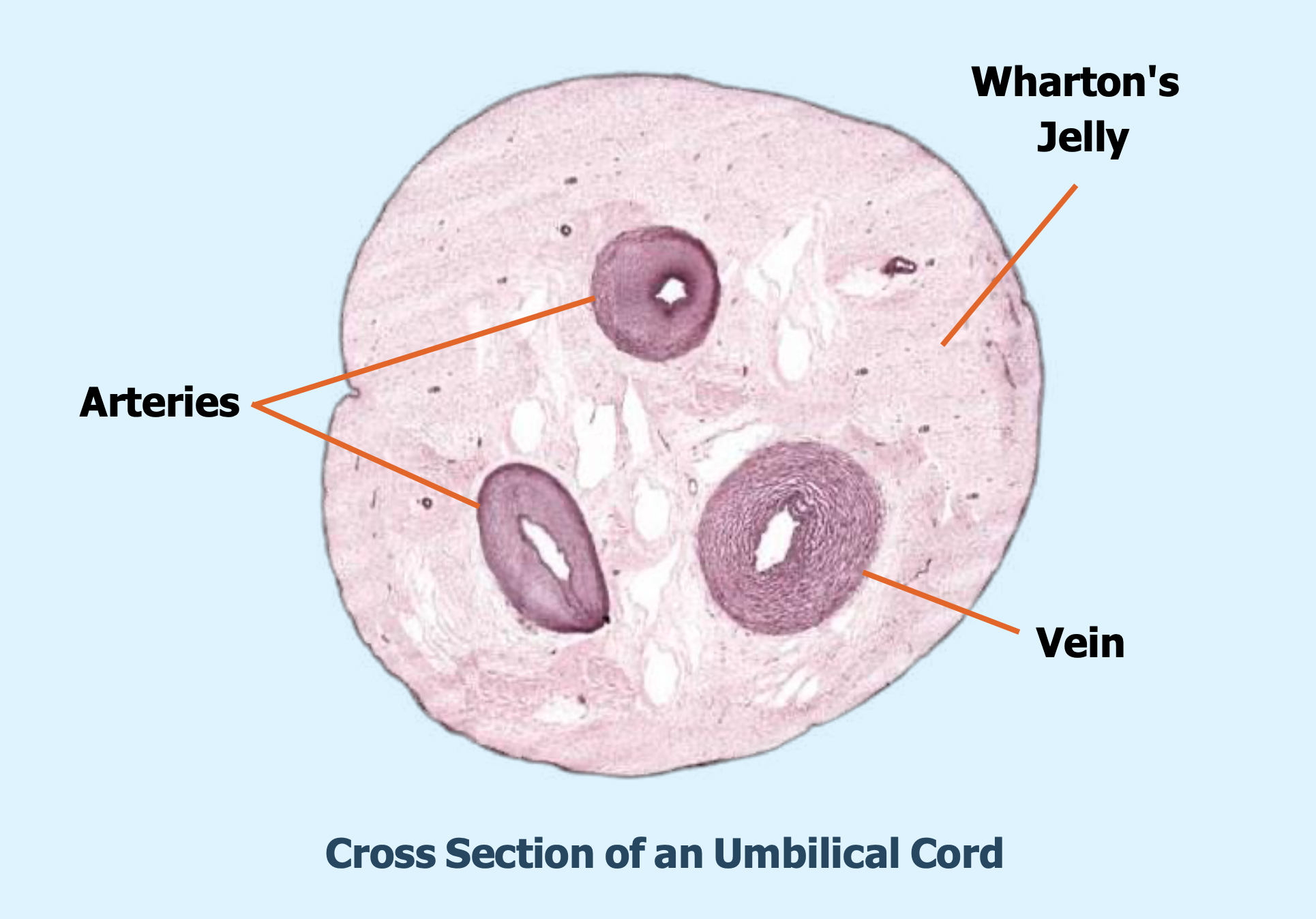
Although MSCs can be isolated from different sources, which include umbilical cord tissue, bone marrow, fat, or amniotic fluid. Our stems cells are obtained from the Wharton’s jelly taken from donated umbilical cords. Sourcing the MSCs from donated cord tissues makes them readily available, safe, ethical and legal.
These highly competent young MSCs have significant potential to be transformed into whatever type of cells that are necessary to supplement a person’s stem cell count after transplantation. They can also replicate at a faster rate, and have the ability to differentiate into other types of cell and multiply, resulting in improved healing effects in the body

Our new facility consists of two state-of-the-art laboratories – a specialised cell processing laboratory and a molecular biology laboratory for genetics and genomics analysis.
Our cell laboratory will be the first privately owned Biosafety Level 2 (BSL-2) laboratory and Current Good Manufacturing Practice (cGMP) in Malaysia to produce CAR T-cells for cancer immunotherapy for solid cancers in the region, as well as stem cell production and other cell engineering services.
The BSL-2 and cGMP laboratory certifications indicate that a facility follows a stringent set of biocontainment standards to protect laboratory personnel, the surrounding environment, and the community.
We have also commenced with our application of three ISO certifications under the Integrated Management System:
ISO 9001:2015 ISO 15189:2014 ISO 17025:2017
The ISO certifications are expected to be obtained by the end of 2021.
Skin Care Professionals?
Get Distributor Price
Contact Us

-
CR+ skincare’s methodology is based on skins structure and functions, cells and systems. We developed our range of skincare treating both the symptom and the cause of compromised skin conditions. All the nutritional ingredients in our skincare is to restore the functions and system of every cells in the skin.
Navigation
Home
About Us
Home Care
Intensive Care
Aesthetic Equipments
Contact Us
Contact
enquiry@cr-plus.com.my
+6016-222 2823
Passion Aesthetic
Malaysia Sdn. Bhd.
T3-19-13A, Menara 3, Jalan Ampang, 50450 Kuala Lumpur
